epoxy acrylate resin
synthesis
synthesis reaction equation of epoxy acrylate resin Figure
The epoxy acrylate resin is made of an epoxy resin and acrylic acid under the action of a catalyst. In order to obtain an epoxy acrylate resin of a high-optical cure rate, a high epoxy content and a low viscosity epoxy resin are selected, and more acrylic groups can be introduced. Therefore, the bisphenol A epoxy acrylate is generally selected E-51 (epoxy value (0.41 ± 0.03) EQ / 100G) or E-44 (epoxy value (0.44 ± 0.03) EQ / 100G); phenolic epoxy resin The F-51 (epoxy value of (0.51 ± 0.03) EQ / 100G) or F-44 (Epoxy value is (0.51 ± 0.03) EQ / 100g).
Catalyst
generally use tertiary amine, quaternary ammonium salt, common triethylamine, N, N-dimethylbenzylamine, trimethylbenzyl chloride, triphenyl Phosphorus, triphenylbin, acetylacetone chrome, tetraethyl bromide, etc., from 0.1% by weight to 3% by weight. Although triethylamine is cheap, but the catalytic activity is relatively low, the product stability is slightly poor; the quaternary ammonium salt is slightly higher, but the cost is slightly higher; the triphenyl phosphorus, triphenylbinds, and acetylacetone have high catalytic activity. The viscosity is low, but the color is deeper.
Corporation
Acrylic acid and epoxy-based open cyclic esterification reaction is exothermic reaction, it is necessary to add a polymerization agent to prevent polymerization of acrylic acid and epoxy acrylate, commonly used The polymerization agent is to hydroxybenzyl, 2,2,5-dimethyl phthal diphenphenol, 2,6-di-tert-butyl phenol, etc., with an amount of about 0.01% to 1% by weight. .
Sub-reaction
Synthesis of epoxy acrylate resin is often accompanied by side reactions, mainly:
behind three side reactions can cause resin crosslinking The gel, so the conditions such as the reaction temperature are controlled when the reaction is improved.
The degree of reaction monitoring reaction is understood by measuring the acid value of the reaction system; the end of the reaction can be measured by the iodine value of the product to understand the loss of the double bond during the synthesis; can also be understood by the product of the product. Epoxy content.
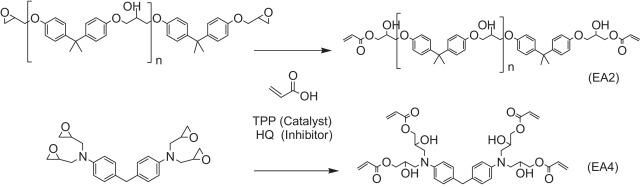
Modified by epoxy acrylate
epoxy acrylate is the most widely used photocurable prepolymer. From the structure, it can be divided into bisphenol A-type epoxy acrylate, phenolic epoxy acrylate, epoxide acrylate, and modified epoxyacrylate. As a body resin, the coating film has good adhesion, chemical resistance and strength, but also disadvantages, such as the flexibility of the cured film, and the brittleness, such as the flexibility of the cured film. Therefore, in order to meet the needs of different fields, the modification of epoxy acrylate (physical and / or chemical) has become one of the research hotspots in this field.
Physical modification is to add nanoparticles in epoxy acrylates to improve performance during curing; chemical modification is an epoxy group or hydroxyl group in epoxy acrylate and other modifications. The functional group in the substance reaction to prepare different properties of modified products.
Physical modification
Inorganic nanoparticle modified epoxy acrylate is a commonly used physical modification method. Commonly used inorganic nanomaterials are montmorillonite, nano-SiO 2 , nano Al 2 o 3 , nano TiO 2 222Et al. The EA can be combined with the nano-inorganic material by hybridization techniques, and the material can be made to maintain the organic polymer film formability and transparency, while having solvent, high hardness and abrasion resistance.
Chemical modification
1) Polyol modified epoxy acrylate
The reaction process of polyol modified epoxy acrylate is shown: < / p>
The essence is an enchain process, mainly by reacting with an epoxy group to access the alcohol's flexible segment into the backchain of the epoxy resin. Due to a rigid group such as a benzene ring, the epoxy resin is high and brittle. In the flexible chain contains a -c-c- and-C-O-bond that can be rotated, the modified epoxy acrylate flexibility will have a certain degree of improvement, and the viscosity is also lowered. However, the chain extended process will also grow the product molecular chain, and the flow resistance increases. When the molecular weight of the polyol is too large, the viscosity of the modified epoxy acrylate will increase. Therefore, the molecular weight and amount of polyol modified polyols should be applied.
2) Acid and anhydride-modified epoxy acrylate
The process of organic acid modified epoxy resin is also an expanded process of epoxy resin, carboxyl group of organic acid The epoxy resin reaction can be introduced into the epoxy resin main chain to prepare a flexible epoxyacrylate.
3) Polyurethane-modified epoxy acrylate
bisphenol A-type epoxy acrylate contains rigid structures such as aromatic ring on the molecular chain, poor flexibility, polyurethane has adhesive Excellent junction, easy to control, excellent segment flexibility, polyurethane segments introduced to the epoxy resin main chain are an effective means of improving epoxy resin performance.
Polyurethane modified epoxy acrylate is mainly divided into two categories: (1) Polyurethane or urethane acrylate is added to the epoxy acrylate photocurable system by physical blending. (2) First synthesis of the prepolymer containing isocyanate, and then reacts with an epoxy acrylate. The phase separation occurs by physical mixing modified epoxy acrylate. Overall, the flexibility of the film after the modified epoxy acrylate was cured.
4) silicone modified epoxy acrylate
silicon polymer - Si-O-key energy (450 kJ / mol) is much larger than -CC-key ( 345 kJ / mol) and -CO-key energy (351 kJ / mol), have the advantages of good thermal stability, oxidation, weather resistance and low temperature characteristics, use it to modify epoxy resins can reduce internal stress, but Increase toughness and high temperature resistance. [
(5) Phosphorus modification
epoxy acrylate has flammable, limiting its application in the fields of microelectronics. For organic coatings, flame retardation is also important, and phosphorus compounds can improve flame retardant properties. When the surface of the polymer is burned, the phosphorus-containing compound can be expanded, the volume increases, and the inner portion of the polymer is from the continued combustion of the flame, thereby increasing flame retardancy.
The primary characteristics of phosphorus modified epoxy acrylate are: In the process of gradually increased, phosphorus-containing group decomposition forming a C-P structure enhances its thermal stability at high temperatures. The ultimate oxygen index of the curing system is improved, and the flame retardancy of epoxy acrylate is improved.
Classification and application of epoxy acrylate resin
bisphenol A epoxy acrylate
bisphenol A epoxy acrylate is imported in oligomers The fastest curing speed, high curing film, high gloss, chemical resistance performance, better heat resistance and electrical energy, plus the source of raw materials, low price, simple synthetic process, therefore extensive application Curing wood, plastic, metal coating or adhesive body resin. However, the bisphenol A epoxy acrylate has a shortcomimulator of the cured film and the disadvantage of brittleness.
Bisphenol A epoxy acrylate contains an aromatic ether bond, the cured product is easy to reduce the chain and produce yellow change, and the chemical reaction format is shown:
phenolic Ephenol acrylate
Phenolic epoxy acrylate is a polyfunctional group acrylate, therefore higher than bisphenol A epoxy acrylate reactivity, more crosslinking density; also possessed and bisphenol A Preferred cure membrane hardness, high gloss, chemical resistance and electrical properties, mainly used as optical cured soldering inks.
Epoxy oxide acrylate
The advantage of epoxide oil acrylate is the price is inexpensive, flexible, strong adhesion, but its light curing is slow, and the mechanical properties are more Difference, so it is generally applied to a photocurable coating with other highly active oligomers.
